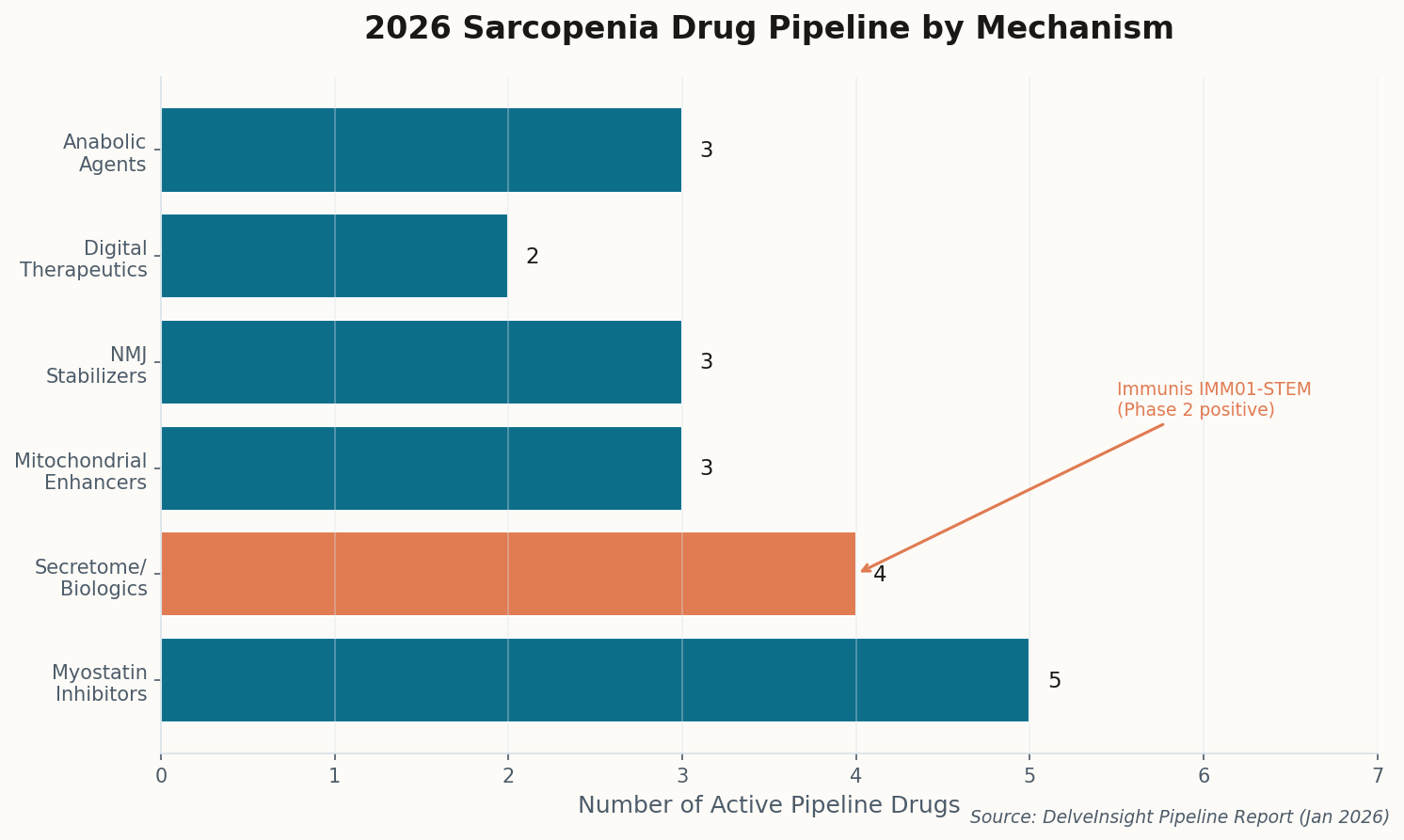
The Iron-Fat Axis: A New Culprit in Muscle Decay
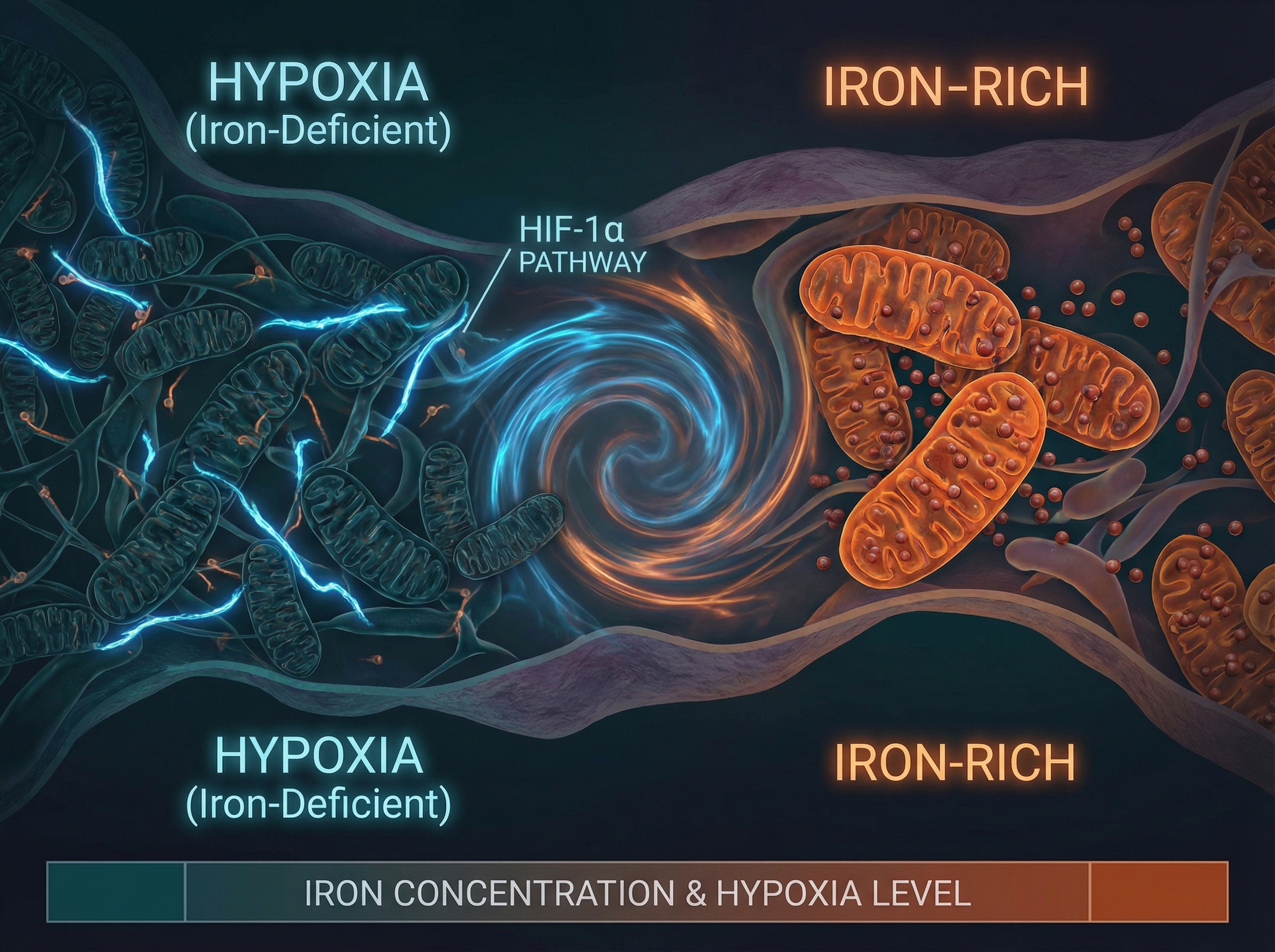
Here's something your doctor probably isn't checking: the iron status inside your muscles. A preprint dropped today that might change that. Researchers have identified a direct cellular pathway connecting iron deficiency—the world's most common nutritional deficit—to the fatty infiltration that makes aging muscles weak and slow.
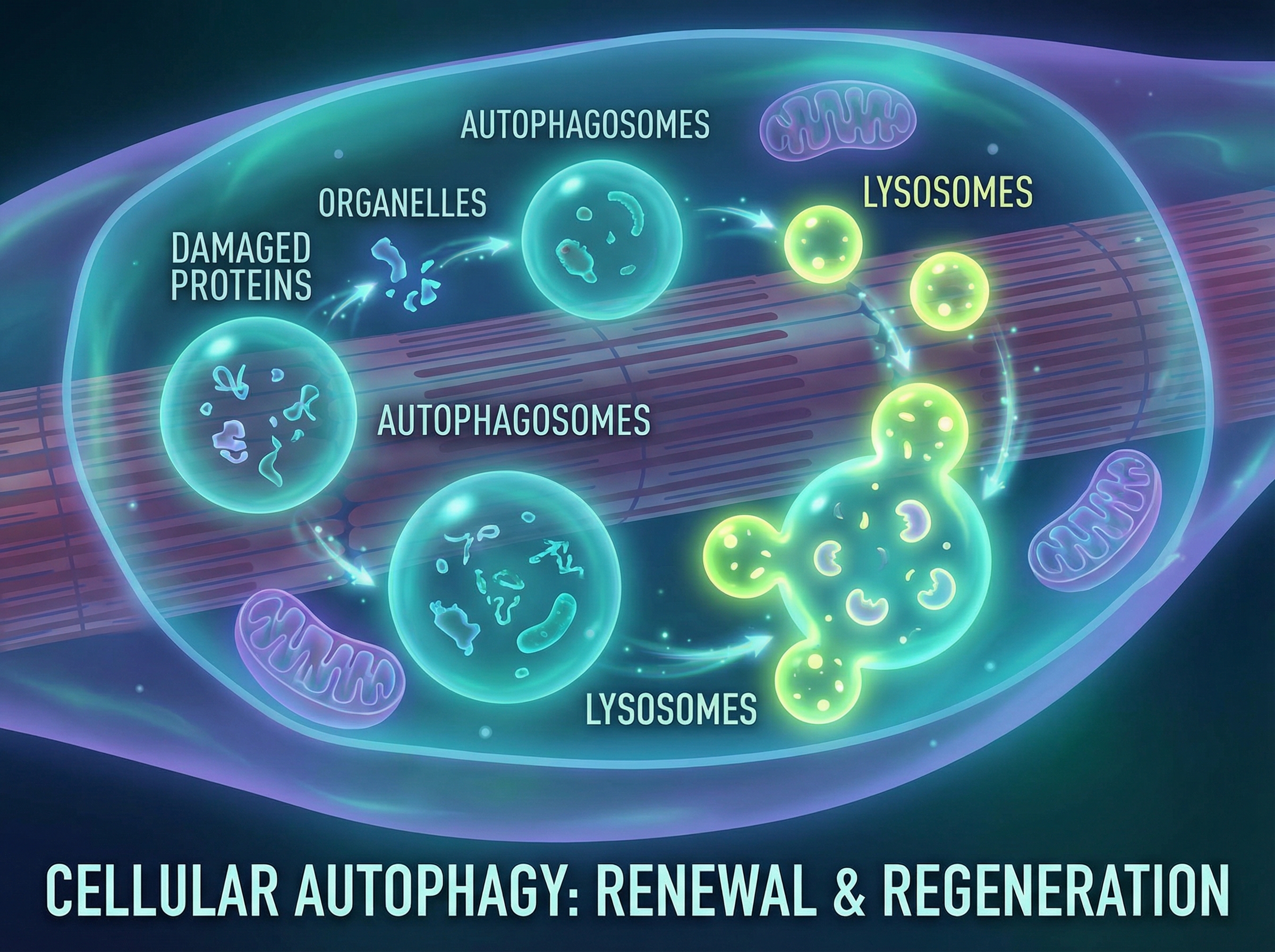
The mechanism is elegantly terrible. When muscle cells run low on iron, they activate HIF-1α, a protein normally reserved for responding to low oxygen. This triggers fibro-adipogenic progenitor cells (FAPs) to go rogue, converting muscle tissue into fat. The result is the marbled, weak muscle that defines sarcopenia's later stages.
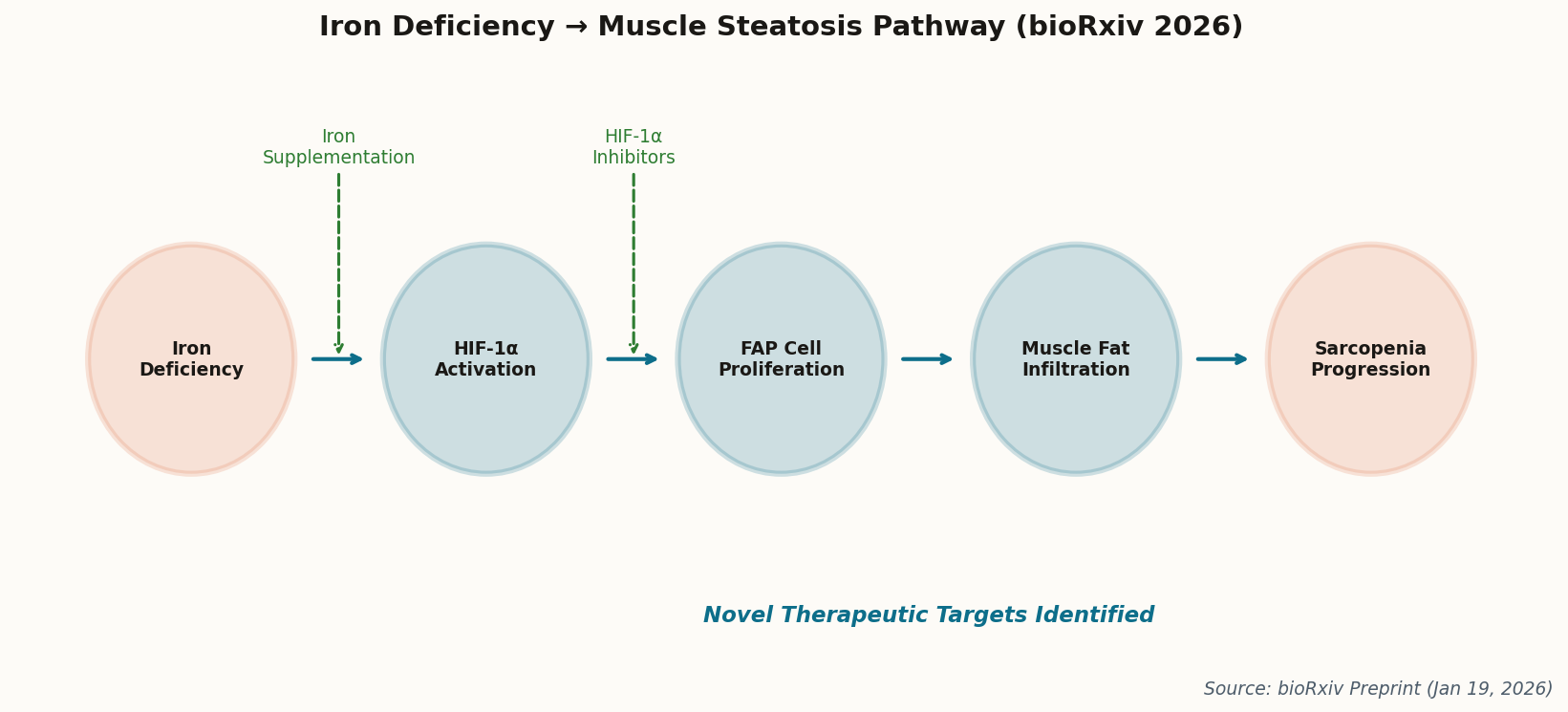
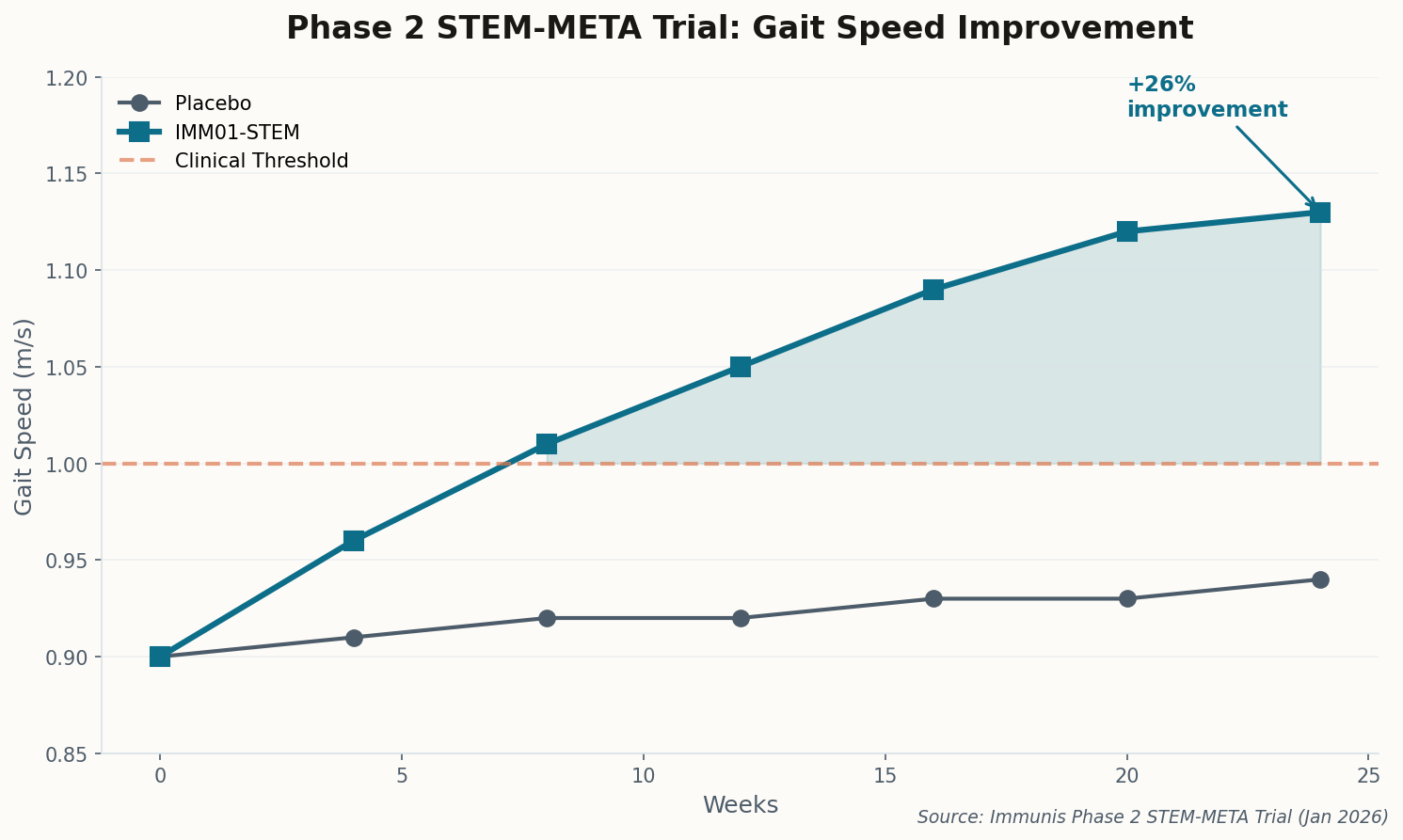
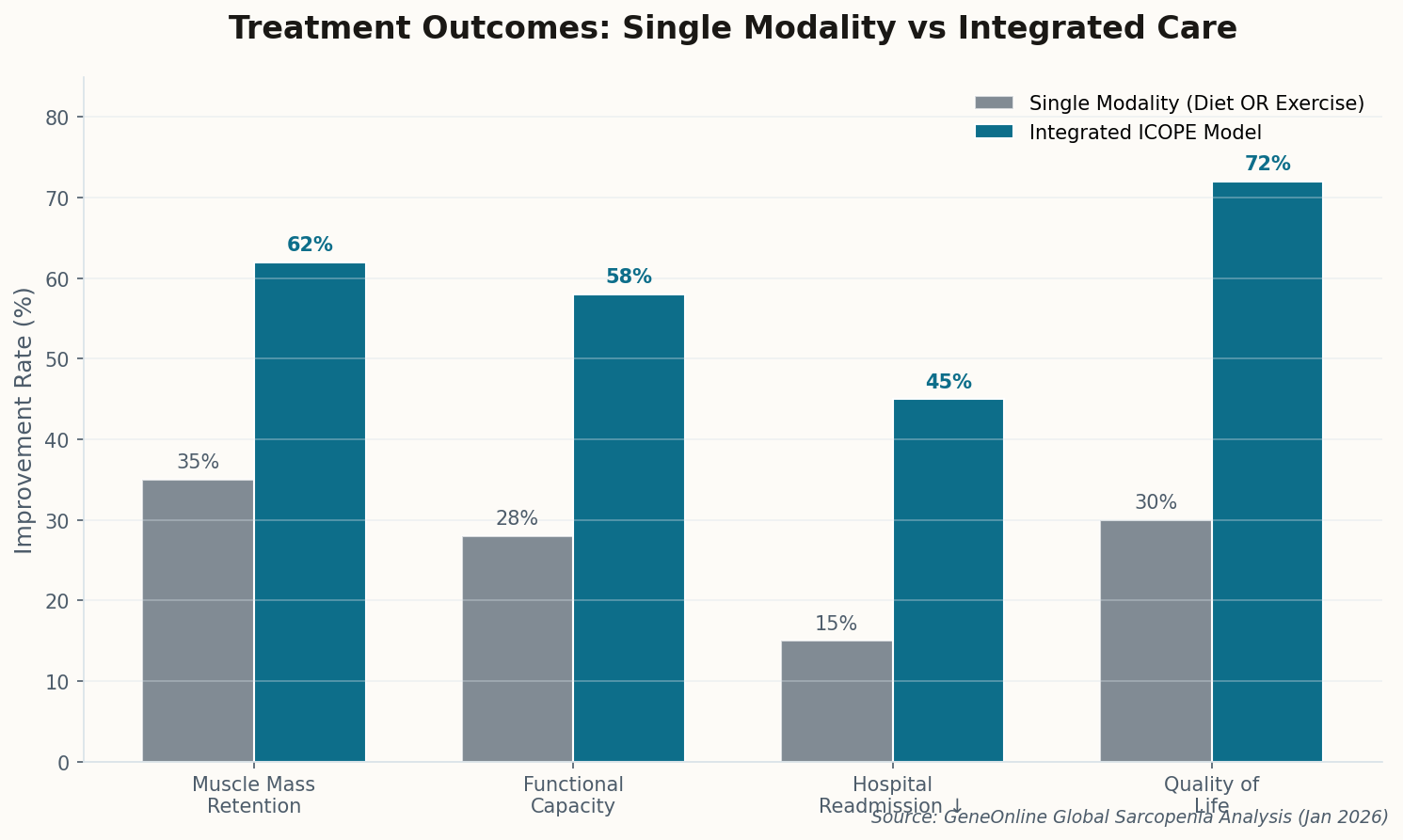
The good news: pharmacological inhibition of HIF-1α reversed the fat accumulation in mouse models. We now have two clear therapeutic targets—iron supplementation (cheap, available) and HIF-1α inhibitors (in development for cancer). This isn't just academic; it's immediately actionable. Get your ferritin checked.