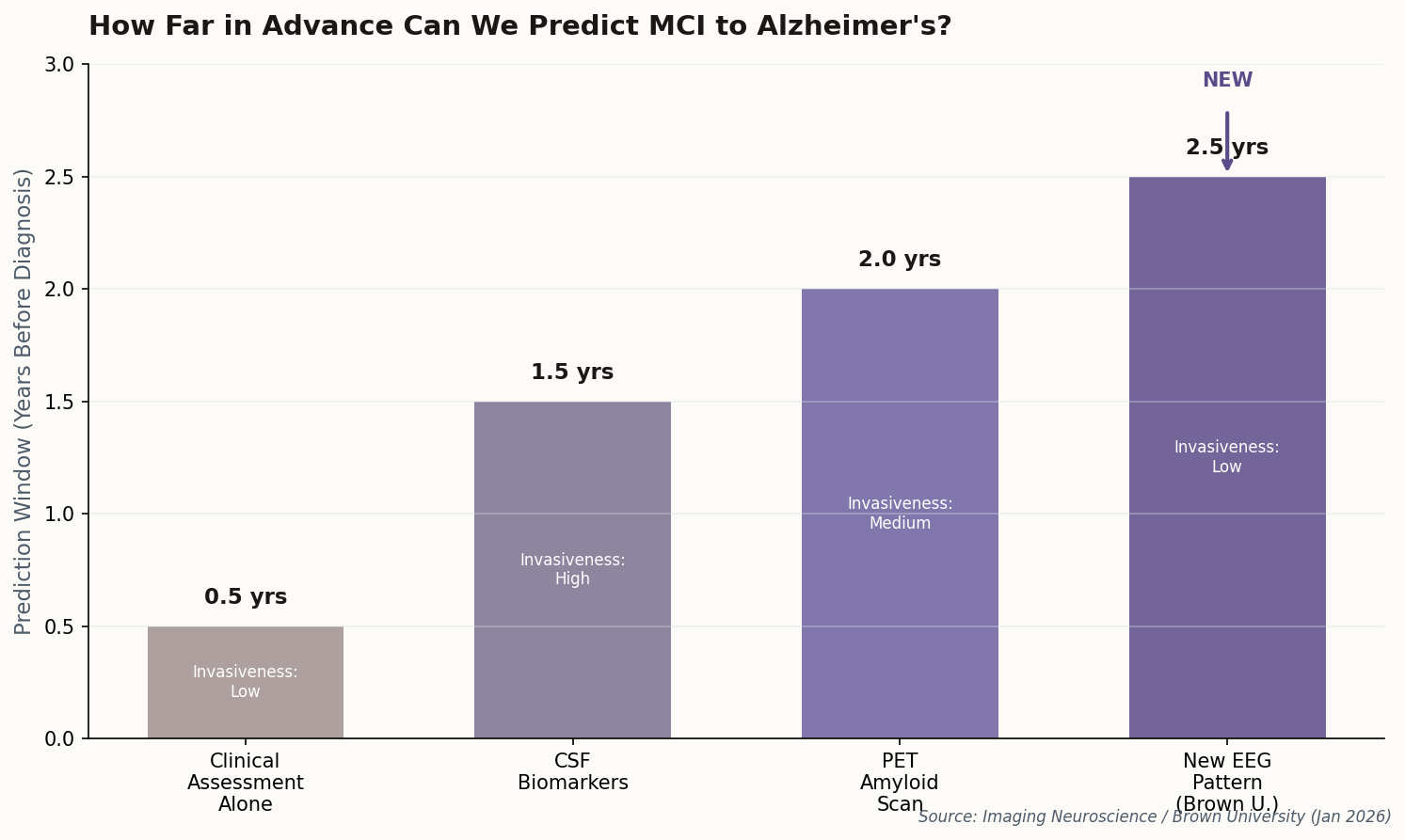
The Second Brain Theory Gets Real
For decades, Alzheimer's research fixated on the brain as both origin and battleground. New research from Arizona State University suggests the war may actually start in your digestive tract—years before you notice anything wrong upstairs.
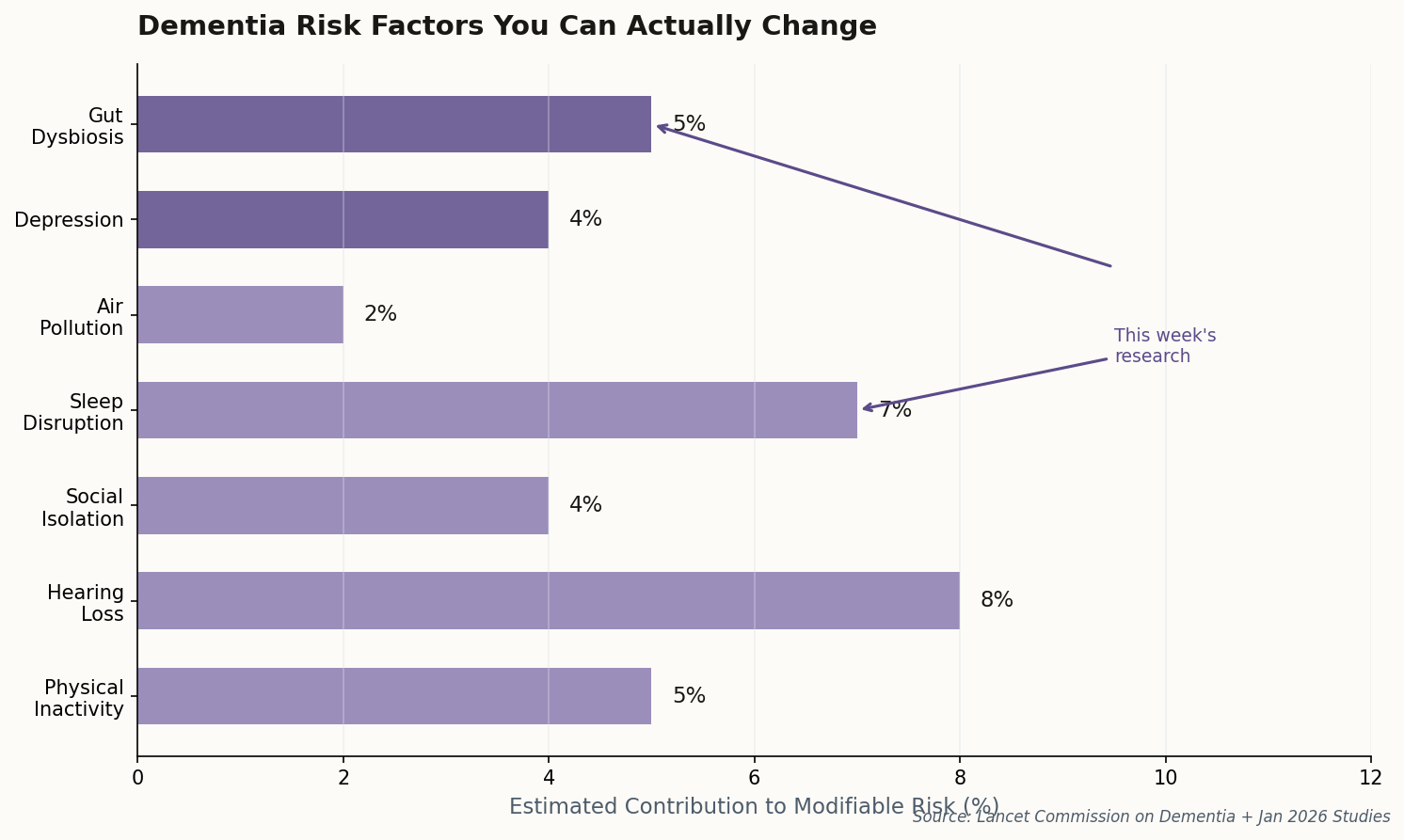
The study identified specific gut microbiome disruptions that precede the inflammatory cascade eventually reaching the brain. Think of it as a biological early warning system we've been ignoring: while researchers hunted for brain-based biomarkers, the gut was already sounding alarms.
The implications are significant. Dietary and probiotic interventions—cheap, safe, and non-invasive—might offer a prevention window we previously thought required expensive pharmaceuticals. This isn't alternative medicine wishful thinking; it's the "body-first" hypothesis gaining hard empirical support.
The takeaway: If gut dysbiosis drives neuroinflammation, then the billion-dollar drug pipeline may be treating symptoms while ignoring the source. Diet might actually matter.